Phage Crusade
A Canadian scientist once harnessed the power of viruses against bacterial infections. In dire times, a new generation of scientists is fighting to do the same.
Jeff Summerhayes knows the drill. The bleak hospital corridors, the calls on the intercom, the IV tubes in his arms dangling from their holders like chandeliers—all have been familiar since Summerhayes’s childhood. But the bug was still in him and all the antibiotics had failed. Now he was lying in a bed at Vancouver General Hospital with his sister sitting beside him, both expecting to hear, once again, that he didn’t have long to live.
Summerhayes has cystic fibrosis, a life-shortening genetic condition that thickens mucus, renders breathing laborious and transforms lungs into prime breeding grounds for bacteria. With a strain of Burkholderia cenocepacia lodged in his lungs for most of the last forty years—a strain that has become extremely resistant to antibiotics—Summerhayes has so far managed to forestall death. A double lung transplant in September 2018 had left him with a fifty-fifty chance of living more than a year, as long as the bug didn’t return. But it did, immediately, and now the doctors were out of options.
For Summerhayes, fifty-fifty odds weren’t bad. He’s no stranger to life with death hanging in the balance: B. cenocepacia is the deadliest of all cystic fibrosis (CF) infections. Diagnosed with CF at birth, and living with this bacteria since the age of sixteen, he’s spent his life pushing beyond expectations. Often operating on about half of most people’s lung function, he has worked on an auto assembly line, shod horses and enjoyed ten years as a whitewater rafting guide on the Ottawa River.
One reason for his tenacity may be that he’s a remarkable member of a remarkable family. His older sister Pam was diagnosed with CF in the 1950s, when the disease was accepted as a childhood death sentence. Research on treatment, not to mention a cure, was nil. Refusing to give up on their daughter, Pam’s parents confronted her doctors at Toronto’s Hospital for Sick Children. They demanded a list of CF patient names so that they could contact other parents to jump-start research; the hospital refused, so the Summerhayes dropped leaflets around the hospital asking other parents to attend a meeting at their house. The result was the Canadian Cystic Fibrosis Foundation, as it was then called, which has raised millions to support research as well as patients and families living with the condition.
Summerhayes’s other sister, Heather Cariou, now sat beside her brother on the hospital bed. Their battling parents—and her vivid memories of Pam, who died at twenty-six—were very much on her mind. Cariou had done her homework. She knew all the antibiotics still in development, including old ones that were being genetically re-engineered, and knew those still might not work. So when the infectious disease doctor arrived for the consultation, Cariou urged her to scour the globe for new, off-the-wall antibiotics. And she asked something she had asked many times before: “What about phage?”
Cariou was referring to phage therapy, a controversial treatment that uses a type of virus to defeat bacterial infection.It makes use of one of the oldest enemies of bacteria found in nature. As Cariou tells it, the doctor’s response was, “We don’t do that here in Canada.” Doctors in the United States had been permitted to administer phage therapy, she explained to the siblings, but only a handful of times: “It’s very experimental, no clinical trial.” Politely, Cariou rejected this caution. “My family doesn’t do ‘We don’t do that here,’” she told the doctor. “My family takes flying leaps—we’ve been forced to all our lives. If Jeff is willing to take the risk, then we’re asking Vancouver General to join him in taking that risk.”
Her persistence began to pay off. First the doctor (whom Cariou calls “an excellent doctor, a caring, well-meaning doctor”) persuaded Health Canada to make an exception to its normal protocols for antibiotics and allow Summerhayes to take one that wasn’t yet fully tested. Four days after the meeting, the antibiotic was flown to Vancouver and the pill drove the bacteria from his lungs.
“Once they got him on his feet I was on-my-knees grateful,” says Cariou, “but I wouldn’t let the phage go. I emailed the poor doctor six studies on phage and links to a dozen others.” Finally, she got what she wanted. The doctor promised that if Summerhayes’s bacteria again develops resistance and his antibiotic eventually fails, she’ll ask Health Canada to approve phage therapy for him. If approved, Summerhayes would be the first person in Canada since the 1940s to legally receive this treatment.
This is true even though phages have already likely saved millions of lives over the decades—and despite the fact that a Canadian pioneered the field, helping save all those lives. But the treatment fell into disrepute and the story behind it is barely known to most Canadians.
Now, it’s being remembered and resurrected, but only because the world’s facing a new scare big enough to outweigh some of the doubts: antibiotic-resistant bacteria like Summerhayes’s, otherwise known as “superbugs.”
Antibiotics were supposed to be the miracle drugs that would bring infectious disease to an end, but superbugs have destroyed that illusion. Maybe now, almost out of options, we’re ready to experiment with something a little more mysterious and a little less within our control. Again, much of this story could play out in Canada; after all this time, Canadian researchers are still poised to be at the field’s forefront, if only they can get the support of their government.
Since the inception of life eons ago, bacteria have been evolving and mutating to counter threats to their survival from various antibacterial agents that occur in nature. This conflict carries on daily in the sea, the earth and in our bodies.
Antibiotic medicine is essentially made of natural antibacterials redesigned to deal a knockout blow to infectious bacteria. But it hasn’t quite worked out that way. Global overprescription of antibiotics and their misuse as preventative measures have spurred superbugs to mutate and defeat virtually all antibiotics. A life-or-death drama is playing out in Summerhayes’s body as his superbug acquires and re-acquires resistance to the array of different antibiotics marshalled against it.
The World Health Organization estimates that antimicrobial resistance (AMR)—the ability of all varieties of superbugs, including bacteria, viruses and fungi, to defeat human treatment—annually causes seven hundred thousand deaths worldwide. This figure is predicted to rise to ten million by 2050, resulting in more deaths than those from cancer. And it’s a number some researchers consider far too low. With even the minimal estimates of AMR damage dwarfing the threat to human life posed by climate change, the WHO has tagged AMR as a major public-health menace. If the trend continues, common strep throat or even a small infected cut could have no cure and might be fatal.
Despite inordinate costs, some pharmaceutical companies are focused on re-engineering existing antibiotics to make them more effective against superbugs. But apart from those initiatives, recruiting phages is one of the few viable solutions remaining to defeat these killer bacteria.
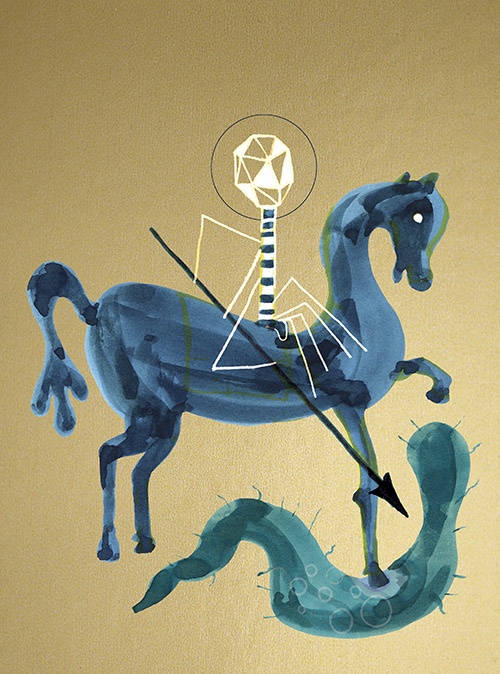
Bacteriophages (literally “bacteria eaters,” called “phages” for short) are viruses that destroy bacteria. Wherever there are bacteria—and human intestines contain billions—even tinier phages exist as well; phages are in fact the most ubiquitous life form on the planet, and probably the oldest antibacterial found in nature. The advantage of phages as bacteria-killers is that, unlike antibiotics that nuke many bacteria in the body—both bad and good—a phage attacks only one species or strain of bacteria.
Jonathan Dennis, a microbiologist at the University of Alberta, is one of Canada’s leading phage therapy researchers. He’s also the only one working on “compassionate use” cases like Jeff Summerhayes’s, where lives are on the brink. After a phone call from the Vancouver General doctor who was treating Summerhayes, Dennis received a sample, or isolate, of the B. cenocepacia threatening him—no longer in his lungs but probably still lurking elsewhere in his body. Dennis then began searching for a matching phage in case the bacteria surfaces again and proves resistant to Summerhayes’s current antibiotic.
Dennis has made the Burkholderia cepacia complex (Bcc)— the group of bacteria including the one that’s been plaguing Summerhayes’s lungs—the focus of his work for nearly twenty years. At the start of his career, he did basic science investigating the molecular biology of pathogenic bacteria like Bcc. But in clinical settings he was struck by how CF patients with that bacteria face extreme and potentially fatal side effects such as spiking fever, severe pneumonia and weight loss. “These patients never know if or when their bacteria will turn angry and try to kill them,” says Dennis. “They’re essentially walking around with a time bomb inside them.”
Their predicament moved him, especially since he saw little improvement in infected patients from their multiple doses of antibiotics. So Dennis resolved to find an alternative treatment. “I wanted to do something significant, that had an impact, not just do what’s been done. There had to be something better that could be used alongside antibiotics,” he recalls. “That’s when I started thinking about phage therapy.”
The modest lab in Edmonton where Dennis spends his days is staffed by six students wearing white lab coats and latex gloves. Like most microbiology research labs, the lion’s share of their funding doesn’t go into fancy equipment. There are lines of test tubes, stacks of petri dishes, a foot-high magnifier and the most high-tech devices, microcentrifuges to separate biological material. Serious gene sequencing gets done elsewhere on campus. In the lab, notebooks—old-fashioned pen and paper to record observations—complete the picture.
But the core of every phage researcher’s lab is its phage bank or library. In Dennis’s case this is a walk-in refrigerator preserving about thirty phages at different stages of preparation. It also contains about two hundred environmental samples from sources rich in bacteria where healing phages might be found: the soil around plant roots, bird droppings and sewage outlets, especially from hospitals where the excrement from recovering infectious disease patients will contain curative phages.
Researchers have their “aha” moments when they watch as, on a petri dish, widening circles of phage devour a bacterial culture. Behind each of those moments lie hours, weeks, possibly years of work—isolating a likely phage, sequencing its genome and determining where and how it attacks the bacterial cell. Once a phage is identified as a match for a certain bacterial strain, more labour is needed to purify it of possible toxins that might trigger a damaging response in the patient. Therefore, the highest cost of phage therapy research is labour: paying investigators and their assistants for all the time required to characterize each phage that might match a superbug.
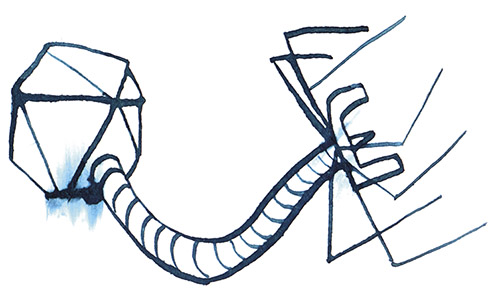
When Dennis views the tiny, individual phages through an electron microscope, what he sees looks like nothing else on Earth: a geometric head, like a lunar lander, perched on incredibly delicate legs. These legs surround a long probe that pierces the bacterial cell and injects the phage’s DNA into the host. In most cases, this hijacks the host’s replication mechanisms, forcing it to produce many copies of the virus at the cost of the host’s life. These phages then burst out of the host cell to attack surrounding bacteria.
Still, the methods Dennis relies upon today—isolating the bacteria and testing phages against them one by one—have hardly changed since the viruses were first discovered over a century ago. The man who co-discovered and named phages was also Canadian: Félix d’Hérelle, who was born in Montreal in 1873. He moved with his family to Paris at an early age and travelled widely in his teenage years. At twenty-four, d’Hérelle returned to Montreal, resolved, with only a high-school education, to become a bacteriologist. After distilling liquor from maple syrup and eradicating locust plagues in Argentina, he ended up again in Paris, at the Pasteur Institute, where he manufactured dysentery vaccines and struggled to find a cure for the disease.
In 1917, he made an interesting observation; he applied a solution from the stools of recovering dysentery patients to a culture of dysentery bacteria and found that the bacteria disappeared. Though not the first to observe the phenomenon, d’Hérelle drew a new conclusion: he believed a virus in the stools had attacked the bacteria in these patients and triggered their recovery. Noting further that the phage in his petri dish spread out to destroy the whole bacterial culture, he also deduced that the virus was reproducing itself in the process of killing the bacteria. The cocksure d’Hérelle was convinced he’d found a cure for dysentery—and a form of microbe that could cure other infectious diseases as well.
These conclusions were a milestone in humanity’s war on bacterial infections. Most infections are routinely defeated by a healthy immune system and occasionally with common plant remedies for infection like garlic and ginger. But unlike those inert antibacterials, phages are alive, and d’Hérelle understood that a bacterial species may have dozens of varieties or strains, each with its phage counterpart.
Evolutionary principles come into play as well. When bacteria are attacked by a phage, those with greater potential for resistance to the phage will be selected for and eventually mutate into a strain that will defeat the phage’s onslaught. This evolution of resistance can take place very quickly, even over the course of a single treatment. D’Hérelle therefore developed “cocktails” with different phages to anticipate possible bacterial evolution during the application of the cocktail.
D’Hérelle’s knowledge of phage biology was basic; genes had barely been named, and molecular biology was not yet born. His goal was to heal, and his approach was pragmatic. When five young people with dysentery recovered after he’d treated them with phages, d’Hérelle’s main concern was not proving beyond a shadow of a doubt that phages were responsible for the cure. For him, their recovery was enough to justify the method and he became its flamboyant promoter.
Once d’Hérelle published his results, interest in phage therapy spread quickly, especially in countries where infectious diseases like dysentery, cholera and typhoid fever were rampant. These were garden-variety pathogens, not the extremely resistant kind evolving later, so his success was partly due to filling a big basket with low-hanging fruit.
D’Hérelle was soon recognized as a pioneer. In 1925 he was awarded the Leeuwenhoek Medal in microbiology, a prize given only once a decade and previously won by his hero, Louis Pasteur. Three years later, d’Hérelle, a passionate socialist, allowed the commercialization of his most effective cocktails but reinvested his share of the profits in his research facility. By 1930 commercial preparations of phages were available throughout Western Europe and North America.
Unfortunately, the treatment’s success planted the seeds for its own downfall. The vast numbers of patients claiming cure by phages overwhelmed the need to examine their biology and chemistry more closely, which meant that when the treatment failed, explanations were sometimes lacking. And with little regulatory control of their contents, the remedies often contained insufficient amounts of phage, or none at all: accusations of snake-oil medicine cast shadows on the whole method.
But the main criticism of phage therapy was the lack of strictly empirical methods of verifying that it was phages and not, for example, a normal immune response that had allowed these recoveries. The one major trial d’Hérelle had conducted in an Indian village during a cholera epidemic—administering phages to one half of the inhabitants and not to the other—cut no ice by the standards of Western medicine.
Meanwhile, d’Hérelle had launched a venture that further alienated him from the medical establishment. In the early 1920s, his student George Eliava at the Pasteur Institute had returned home to Georgia, then part of the Soviet Union, to establish an institute for microbiology in the capital, Tbilisi. The two scientists were close friends, and d’Hérelle visited the institute in the early 1930s to help educate the staff about phages.
D’Hérelle and Eliava planned to set up a separate institute just for phage therapy, also in Tbilisi. The Soviet government approved their vision and construction started. But Eliava fell foul of one of Stalin’s henchmen and was executed in the Great Purge of 1937. Crushed by his friend’s death and disillusioned by the brutal reality of Stalinism, d’Hérelle never returned.
By now the West was not a safe haven either. D’Hérelle’s alleged communist sympathies, combined with the lack of proper clinical trials and supporting evidence for the efficacy of phages, had a chilling impact on this first wave of phage therapy. Once penicillin and other antibiotics were readily available after World War II, the treatment was mostly abandoned, except in France, Poland and the Soviet Union. Although nominated dozens of times for the Nobel Prize in his lifetime, d’Hérelle ended up a footnote in the history of twentieth-century bacteriology.
The controversial treatment began a slow return to Canada thanks, in part, to a painful accident. In 1996, a Toronto stand-up bass player named Alfred Gertler fell and broke his ankle so badly that the bones protruded from his skin. When the cast was removed, the bones had mended but were severely infected. Eventually, the infection spread so deep that no antibiotics could reach it, leaving an open wound that refused to heal: all the doctors could offer was amputation.
“I was told to give up hope, but I didn’t,” he says. He’s used to fighting; “as a musician, even on a good day you just exist from one day to the next.” In January 2000 he visited a friend in hospital. On the table beside the bed was a New York Times article titled “A Stalinist Antibiotic Alternative” about how phage therapy was practiced in the former Soviet republic of Georgia. “I grabbed it and ran,” Gertler says, reading as much as he could about the treatment.
The article described how the Eliava Institute had weathered its founder’s death, continuing basic research and treatment, while in the West phage therapy had withered away. The institute had also built up a large library of phages from the standard phage cocktails d’Hérelle had brought from Paris. During World War II the Soviet army had relied heavily on phages manufactured at the institute and in the Soviet Union to prevent and treat dysentery and gangrene. After the war, the Soviets, unable to afford most Western-made antibiotics, churned out phages from the institute in vials and tablets to treat a wide range of illnesses from strep throat and whooping cough to cholera.
But once Georgia declared its independence in 1991, Russia stopped funding the institute. Several years of civil war followed, and most of the institute’s equipment was privatized or destroyed. Toilets didn’t work and the labs were in disrepair—only the dedication of the staff kept minimal services going. Worst of all, electricity was cut off for most of the day, so in the summer the refrigerators could no longer preserve the phages and researchers took them home to keep cool.
Meanwhile, in North America, where phage therapy was dormant after World War II, interest shifted to phages’ relatively simple physiology. Phages became a major focus in the burgeoning field of molecular biology, leading to the discovery of DNA and the double helix. One of the top researchers in phage biology was Elizabeth (Betty) Kutter at Washington State’s Evergreen State College, who specialized in phages that attack E.coli bacteria.In the 1970s and 1980s, Kutter built up a significant phage library and body of knowledge.
Her efforts allowed phage therapy to take root again on this continent. In 1991, Kutter’s research took her to Moscow, where she kept hearing about the beautiful, mountainous Soviet republic of Georgia, still home to the Eliava Institute. She visited and was struck by the institute’s healing potential. “I was immediately drawn to it,” she says, and she’s returned almost every two years ever since. Coming back to America, Kutter began promoting phage therapy to anyone who would listen.
Gertler devoured all this information in the Times article, especially the reference to Kutter’s biannual international phage biology meeting, which was being held in July of that year in Montreal. Gertler scraped together the money to go and register as the only non-academic attendee. He met Kutter, who urged him to go to Georgia, and two researchers at the conference, one from the Eliava Institute and one from an Israeli biotech firm, who both offered to find matching phages. Gertler was doubtful, but he ducked into a washroom and took swabs from his infection, which the two took home to compare with phages in their libraries.
Both found matching phages: the Eliava researcher invited him to Georgia for treatment while the Israeli sent him the phage solution. Gertler knew that only a qualified doctor could administer the phage to the gaping hole in his foot. In Toronto, he sent a request to Health Canada for compassionate-use approval, but it failed on the grounds he wasn’t dying. He was reduced to hobbling around to doctors’ offices lugging the phage and supporting documents to plead his case.
But the doctors were spooked. A year before, in Toronto, a woman had acquired an antibiotic-resistant infection in hospital and had been secretly treated with phages. The infection disappeared, but she died from other complications. The doctors involved risked censure from the College of Physicians and Surgeons, and possibly losing their licences, for administering a drug that didn’t have regulatory approval. When the case was leaked to the press, the health authorities immediately threatened legal action and the doctors disappeared from public view.
With all doors closed to him, Gertler finally agreed to Kutter’s advice. She accompanied him as he became the first North American to take the midnight plane to Georgia for phage therapy treatment. In Tbilisi, the “Pyophage” cocktail dripped onto his foot by the Georgian doctors was essentially the same mix d’Hérelle had brought to the institute in the 1930s, regularly maintained and updated every six months, as d’Hérelle had advised.
One year from the time Gertler first read about phages, his foot, deemed a dead end by Canadian doctors, had fully recovered. “I’m very grateful to Betty and the Georgians,” says Gertler. In the Georgian language the word for fate or luck is “bedi,” and the doctors took the similarity to Kutter’s first name as a positive sign. “I’m standing here, I’m not in pain, I’m healthy,” says Gertler, who still plays stand-up bass. “I was cured of something that was not officially curable.”
Despite the fact that phage therapy had been first used by a Canadian, the hangover from the high-flying d’Hérelle years lingered. Resistance to anything new, plus inadequate basic science funding—not to mention objections from the medical establishment—has stymied generations of phage therapy investigators in Canada.
It hasn’t been for lack of trying. As news of the superbug invasion spread in the 1990s, a second wave of interest gathered momentum in Canada as well. Patients like Gertler were demanding better treatment, and several biotech firms exploring phage therapy sprang up in Montreal and Ottawa. Around the same time, a young microbiologist named Sylvain Moineau took a teaching position at Université Laval in Quebec City. Much of Moineau’s work was applied research, investigating phages that destroyed the fermenting bacteria in dairy products like yogurt—“bad” phages, in other words, from a human viewpoint, not the “good” phages that healed Gertler’s foot.
Moineau found a major phage resource at Laval. Canada was home to one of the world’s largest phage libraries outside Georgia: the Félix d’Hérelle Reference Centre for Bacterial Viruses, founded in 1982 by the late Hans-Wolfgang Ackermann, a renowned microbiologist who trained at the Pasteur Institute. Researchers could call Ackermann and he would send out a phage, along with its characterization, at minimal cost. During the centre’s early years, there had been few Canadian researchers focused on phage therapy to take advantage of Ackermann’s largesse, but that was changing.
Brett Finlay, a microbiologist at the University of British Columbia who served on the scientific advisory board of a federal agency for health research, pushed for a response to the superbug crisis. In 2005, the agency—called the Institute of Infection and Immunity—heeded Finlay’s advice by allocating millions of dollars into a research iniatitive called “Novel Alternatives to Antibiotics.” Aware of Canada’s widespread ignorance and antipathy around phage therapy, the agency wisely tucked research into the treatment under the initiative’s protective wing.
The Novel Alternatives to Antibiotics project funded five years of research on phage therapy from three teams headed by Moineau, Alan Davidson at the University of Toronto, and Dennis. The new initiative “was the most prescient idea anyone could have imagined at the time,” says Dennis. “That large grant fostered a lot of the research results we’re using today in the treatment of patients.”
Typical of those results was a landmark paper by Dennis’s team—which Heather Cariou later gave the Vancouver General doctor—showing that phages sprayed into the lungs of mice infected with B. cenocepacia significantly reduced the mice’s infection rate. But when the funding ended after 2012, the hope that it would lead to more grants did too. Under Prime Minister Stephen Harper, the federal government made substantial cuts to science during this time. The researchers have declined to talk about specific details of why their funding ended.
Also in 2012, the d’Hérelle Reference Centre at Laval, with its collection of five hundred phages matching over 125 bacterial species, also lost most of its funding. As curator of the centre, Moineau had to decide if he should continue its role as “a service to the community” or shut it down completely. He kept it open by increasing the fees for each request, which could range from one to fifty phages—and then watched the number of annual requests reduce by half, with no funding agency rushing in to help out. “Trying to get grant money for what looks like mundane characterization is a tough row to hoe,” says Dennis. “The science is not, perhaps, sexy enough to get normal grant funding. But it’s essential.”
Dennis himself struggles to overcome opposition with every grant proposal he writes. These funding woes understandably frustrate Heather Cariou, as she watches the treatment that could save her brother—and countless others—go unsupported. “This man is doing breakthrough research and he’s not getting properly funded,” she says of Dennis. “My God, what’s wrong with you, Canada?”
The irony is that the mundane sometimes turns up a diamond. “Big discoveries in biomedical science are usually unexpected, but it isn’t luck,” says Davidson. “These discoveries emerge through the process of doing good science: asking good questions and pursuing the answers.” While working on phage therapy under the Novel Alternatives to Antibiotics initiative, both his lab and Moineau’s chanced on significant insights into the genes in bacteria, called CRISPR-Cas, that ward off phages. These genes are now at the investigative heart of genome-editing research on a global scale.
Another major hurdle is regulators. Physicians and researchers had petitioned the US Food and Drug Administration and Health Canada to adjust their clinical trial requirements to take into account the special problems raised in treating humans with phages. A standard clinical trial has four phases and can involve hundreds or thousands of patients and control participants.
But the regulators were making little progress. They were especially troubled by the fact that, unlike antibiotics, both the phage and the host bacteria often evolve during treatment making each case unique. One basic requirement in a standard clinical trial is consistent application from patient to patient, but administering phages to large numbers of people makes this virtually impossible.
Moreover, current regulations would stipulate that each phage in a phage cocktail be considered a separate drug needing its own clinical trial before approval. The costs would be prohibitive, considering that it’s not unusual for a company to spend $1 billion on a single new antibiotic from discovery to market.
These roadblocks were major disincentives to the commercialization of phage therapy. But the biggest deterrent was that phages in their natural state, like genes, are considered to be alive and therefore cannot be patented. Although later research found ways to navigate this problem, the chances for large profits in phages initially looked unappealing.
Given all these obstacles, not just Canadian biotechs but almost all the second-wave North American phage therapy startups have failed to advance beyond even the first phase of a full-blown clinical trial. As a result, there was—and still is—no ironclad proof of phages’ efficacy, according to North American regulatory standards. “I’m hesitant for people to push hard for big trials of phage therapy at this point, because they may be destined to fail,” concludes Davidson. “They can make people think it isn’t doable.”
Different critics have different theories of exactly why Canada continues to lag. In 2016, Toronto-born epidemiologist Steffanie Strathdee was in despair as her American husband, Tom Patterson, fought off death in San Diego, infected with antibiotic-resistant bacteria nicknamed the “Iraqibacter” for its prevalence among American soldiers stationed overseas. Strathdee, formerly active in HIV/AIDS research and treatment centres in Vancouver, had heard about phage therapy in Georgia and now resolved to try it.
“It was going to be one in a million or even less that we could save him,” recalls Strathdee. “I just felt like I had to try.” With help from researchers across the continent, including one Texas grad student who slept in her university’s lab for two weeks trying to find a matching phage, they succeeded: after massive doses of antibiotics and three separate phage cocktails, Patterson recovered and Strathdee became a phage crusader.
In 2018, she co-founded the American non-profit Center for Innovative Phage Applications and Therapeutics (IPATH). Canadian patients with superbug infections and no recourse in Canada began reaching out to IPATH for help. In a blunt Globe and Mail op-ed in March 2019, Strathdee castigated Canadian funding agencies for not supporting phage therapy research, noting the financial doldrums holding back Dennis and the d’Hérelle Centre: “Canada should take a leading role, not a back seat, in the prevention and treatment of AMR and should be scaling up research and programs to address it,” she wrote.
Strathdee argues that Canada has lagged in funding phage therapy research partly because it has simply failed to catalogue the enormity of the need for it. “Without the scientists really knowing the scope of the problem, the public doesn’t know either,” she says. “And without the public knowing, there’s no impetus for dedicating research support to that problem.” Finally, Canadian health authorities are trying to fix that by quantifying the superbug threat. An expert panel struck by the Canadian Council of Academies to determine the socioeconomic impact of AMR in Canada recently reported that 26 percent of all antimicrobials initially prescribed for infections in 2018 proved to be ineffective, and by 2050, the panel estimates that figure will rise to 40 percent.
Meanwhile, researchers are sounding alarms about their lack of support. Davidson at U of T says the meagre funding is “becoming a real disaster,” as he increasingly sees good researchers, with excellent proposals, “giving up.”
But Canadian authorities have few updates on their phage therapy plans. Elisabeth Pagé, assistant scientific director at the Institute of Infection and Immunity, says the institute’s strategic plan for 2021 to 2025 “will provide leadership for AMR research, including potential alternatives to antibiotics.” Speaking broadly of AMR as a whole, the Public Health Agency of Canada notes that it has spent over $200 million in the last decade on the issue while sending more than $9 million to the World Health Organization. Ottawa “continues to take action, both domestically and abroad, to address this complex issue,” says spokesperson Anna Maddison.
Canada could take a cue from other countries whose initiatives are riding the third wave of phage therapy. In 2018, Belgium became the first in Western Europe to officially undertake phage therapy without requiring extensive testing in clinical trials: pharmacists there can now sell phages upon prescription and approval by a physician. Cost projections for the plan also ensure that researchers’ work of characterizing and purifying phages is adequately compensated. Kutter believes the Belgian approach would also work in Canada if this country had a centralized system for manufacturing and testing the phages.
Two new and very hopeful methodologies recently helped save the life of a fifteen-year-old CF patient in the United Kingdom. The first was a holy grail: genetically engineered phages, two of which were successfully used in the girl’s treatment. Engineered phages, unlike phages in their natural state, can be patented along with other ways to replicate phages’ power such as synthesizing their bacteria-destroying enzymes. They are of great interest to the struggling biotech firms, and by the time these products are ready for clinical trials the regulatory regime may be more flexible.
The other breakthrough in this case relied on crowdsourcing. The third phage used in the girl’s treatment was found in 2010 in the soil scraped from a rotting eggplant by an undergrad student in South Africa. Her discovery stemmed from a novel project at the University of Pittsburgh that encourages any interested undergraduate, or even high-school student, to locate phages and do basic characterization of their properties. Dennis and Moineau have proposed a similar project, dubbed Phage Canada, three times in recent years—but all of their grant applications were rejected.
Maybe it’s the grim risks of not following through that will ultimately motivate change. The Canadian Council of Academies panel reported not just on superbugs’ impact on Canadians’ health, but on their GDP, finding “frightening” numbers, says the panel’s chair, UBC microbiologist Finlay. If the idea of countless deaths doesn’t make phage therapy acceptable, perhaps dollars will.
The UN’s most recent report on AMR says an unprecedented effort by all nations is required to avert disaster. Bacterially infected patients are dying daily by the thousands in high-income countries, and by the tens of thousands elsewhere. Dennis and Strathdee both believe superbugs are the most urgent threat to humankind. “Climate change can be terrifying and very real,” says Dennis, “but long before we succumb to climate change, multidrug resistant bacteria will decimate us.”
Dennis, for one, hasn’t given up. With some interested doctors, he has formed a phage therapy working group whose aims include a limited clinical trial of ten patients—though, to Dennis, even one would be worthwhile.
And Jeff Summerhayes’s case is drawing increasing media attention, a situation the patient himself finds odd. “I’m becoming the phage therapy spokesperson for Canada, and I may not even get to try it,” he jokes. Summerhayes has passed his one-year deadline in good health; these are “million-dollar days,” he says, and he hopes to drive across the country in his Jeep next summer, motorcycle in tow.
Regardless of the outcome of his battle with B. cenocepacia, Summerhayes says he has no doubts about the value of his case. “If I can, by speaking up, help somebody else get phage therapy that will save their life, that would be absolutely amazing.”